

Tackling Nightmares
Understanding NightWare and its role in treating severe nightmare disorder. See how this FDA-cleared digital therapeutic (Class II Device) is transforming the standard of care for patients suffering from traumatic nightmares.
The Hidden Crisis
of PTSD patients experience nightmares that drive a 2-5x higher rate of suicide and 7-10x medical costs.
The Cascading Impact of Untreated Nightmares
Nightmare Disorder is not just sleep disturbance—it is a critical driver of severe psychiatric and functional comorbidities. Clinical evidence links frequent nightmares to:
- Suicide Risk: Significantly increased risk of suicidal ideation and attempts, independent of other symptoms.
- Mental Health Decline: Exacerbates anxiety, depression, and PTSD severity, creating a vicious cycle of distress.
- Cognitive Impairment: Causes daytime brain fog, memory issues, and reduced executive function due to fragmented REM sleep.
- Functional Collapse: Leads to strain on relationships, reduced work productivity, and social withdrawal.
Current Standard of Care Fails

"I would wake up mentally and physically drained. I would wake up drenched. My wife actually had to physically wake me up during the middle of the night because of emotional reactions - yelling or screaming or shaking."

Market Opportunity
The market remains highly fragmented. Traditional methods consistently fail, creating a massive opportunity for a scalable, digital solution.
Why Traditional Approaches Fail
Non-pharmacological, closed-loop intervention with real-time response. Immediate deployment and algorithmic personalization.
Expert Insights & Clinical Perspective
Hear from medical professionals and see why NightWare is poised to become the standard of care for nightmare disorder.

Addressing an Untreated Niche
A doctor's perspective on why NightWare fills a critical gap in treating nightmare disorder where traditional therapies have failed.

The New Standard of Care
Explore the potential for NightWare to transform the treatment landscape and become the primary intervention for patients suffering from severe nightmares.

Patient Stories
Hear directly from some of the 2,000 lives changed by NightWare's technology.

Restoring Hope: A Veteran's Journey
See how NightWare helped reclaim sleep and quality of life.




Understanding Nightmare Disorder
Nightmare Disorder is a serious, debilitating condition that affects millions. Learn more about the science and the impact.

What is Nightmare Disorder?
An in-depth look at the clinical definition and impact of Nightmare Disorder on patients' lives.

The Science of Sleep
Understanding the physiological mechanisms behind sleep disturbances and how NightWare intervenes.
The NightWare Advantage
A revolutionary digital therapeutic that interrupts nightmares without waking the patient.
Stress Index & Intervention
Real-time biometric monitoring
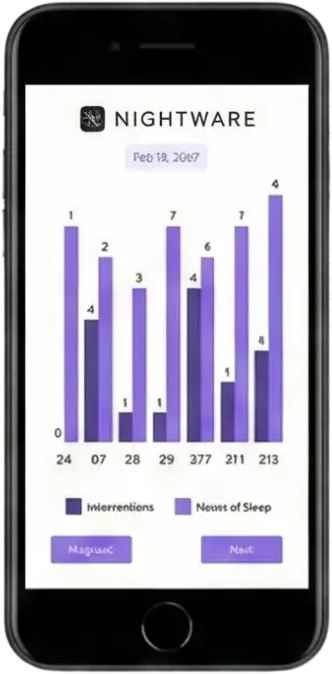

Sleep Quality Index
Weekly progression analysis
Complete Therapeutic Platform
Only FDA-Indicated Nightmare Therapy
Patented algorithm identifies and interrupts a nightmare in real time for better sleep quality. First mover advantage with 2000+ patients since 2021.
4X Market Expansion Ready
Expansion opportunities into REM Behavioral Sleep Disorder, Perimenopausal Sleep Disturbances, and moderate nightmares. Flexible business model.
AI Learns From Every Night
As NightWare scales, its AI enables seamless expansion, continuous personalized therapy, and high-value data for platform improvement.
How NightWare Works
NightWare uses Apple Watch sensors to monitor heart rate and movement during sleep. When a nightmare is detected, the device delivers gentle vibrations to interrupt the nightmare without waking the patient.

A Seamless Nightly Routine
Ensure the NightWare Apple Watch and iPhone are fully charged
Put on the Apple Watch
Just before going to sleep, start the NightWare software application
Go to sleep
Please see the full Instructions for Use for additional information.
The Transformation
"Within three nights, I woke up and realized something was off. I don't remember having any dreams, but I feel extremely rested for once."

Advancing Trauma Care
NightWare is more than just a sleep aid—it's a critical advancement in trauma care for veterans and patients worldwide. Discover how our technology is reshaping the future of sleep medicine.

Proven Traction
Our early traction demonstrates product-market fit with strong unit economics and real-world validation.
Product/Market fit validated
Consistent real-world success
For patients in NightWare's patient management program
Clinical Validation
Statistically significant improvement in sleep quality when patients enroll in patient management program and use NightWare at least 50% of the time.
DOI: https://doi.org/10.5664/jcsm.10338
636 Military Personnel Study
DOI:10.26502/jppd.2572-519X0199
Leadership Team
A world-class team of experts in digital therapeutics, sleep medicine, and medical device commercialization.
Executive Leadership

Matthew Tucker
Chief Executive Officer
20+ years of P&L and portfolio leadership of medical devices and pharma at Baxter, Mylan, startups. Innovation and strategy roles at Highmark Health. Author, The Ten MedTech Commandments.

Dr. Brian Robertson
Chief Medical Officer
25 years in military medicine. Former Chief, Sleep Disorders Center, Walter Reed National Military Medical Center. Researcher, educator, and lecturer in sleep medicine.

Patrick Lynch
Chief Product Officer
25+ Years Product Development and Global Expansion. Multiple US Patent Holder. Global Marketing Roles @ Align, Grifols, Baxter.

Spencer Levy
Chief Operating Officer
25+ Years Operations Leadership Experience. 17 Years Medical Device - Healthcare Management Experience. Operational Process, Strategy & Supply Chain Expertise.
Board of Directors

Jerry Mattys
Board Member

Mike Ott
Board Member

Grady Hannah
Board Member

Matthew Tucker
Board Member
The Raise
We're seeking strategic partners who understand the transformative potential of digital therapeutics.
Use of Funds
Why Now?
NightWare is at a critical inflection point with multiple converging opportunities:
- Clinical Data Inflection
- VA Innovation Pilot
- International Licensing
- Hardware Transition

